Contents
Introduction
Calcium is a divalent element of the alkali earth metal group of the periodic table. The total body content of calcium is around 1kg, 99% of which is located in the bone in the form of calcium phosphate.
In addition to its role in providing the hard, mineral component of bone, calcium also functions as a second messenger and is vital for synaptic transmission and muscle contraction. Calcium also affects the stability of the membrane potential of electrically excitable tissues.
The role of calcium in the function of electrically excitable tissues requires the level of calcium in the blood to be maintained in a narrow range. In most laboratories this range will be given as around 2.1-2.5mM. Much of the metabolism of calcium is concerned with keeping calcium levels within this range.
Corrected Calcium
Approximately half of the blood calcium is bound to albumin and the other half is free (ionised). Only the ionised form is biologically active and exerts the electrical effects mentioned above. In patients who have a low albumin level, the proportion of calcium that is bound to albumin is reduced simply because there is less albumin around to do the job. In these patients, measuring the calcium level alone will be misleading: The ionised calcium level can be normal but the reduction in bound calcium will give a total calcium level that is low.
While it is possible to measure the ionised calcium specifically to address the problem posed by hypoalbuminaemia, most laboratories instead supply the results of a calcium assay as calcium and corrected calcium. The corrected calcium allows for the hypoalbuminaemia and calculates what the total calcium would be if the albumin level was normal.
The principle behind the calculation of the corrected calcium is that every 1g of albumin per litre can bind 0.02mmol of calcium. The normal albumin level is taken as 40g/L. This yields the following formula.
Corrected calcium = Raw calcium + 0.02(40 - patient's albumin level)
The calcium concentration is measured in mM and the albumin levels in g/L.
Intake, Excretion and Distribution
The source of calcium is the diet. A standard diet will provide around 1g (25mmol) of calcium per day, of which the gut absorbs around 350-400mg. The remainder of the dietary intake is lost in the faeces without ever being absorbed. However, of the 350-400mg that is absorbed, 200mg re-enters the lumen of the gut in the secretions of the intestines, so the net intake per day is actually 150-200mg.
The renal excretion of calcium largely balances the net intake of 150-200mg per day. Small quantities of calcium are lost in the sweat.
Bone stores over 99% of the total body calcium (1kg). The extracellular fluids (including the blood) contain around 900mg. The resting intracellular concentration of calcium is very low.
The calcium within the bone is not locked away in a static, unavailable form. Despite its seemingly hard, immutable nature, bone is actually a dynamic tissue that is in a constant state of remodelling and turnover. This activity includes the interchange of around 500mg per day of calcium between the bone and the extracellular fluid. Modification of this interchange is one of the key mechanisms by which blood calcium levels are regulated.
Some aspects of calcium metabolism are linked to those of phosphate.
Parathyroid Hormone
Parathyroid hormone (PTH) is a peptide hormone which contains 84 amino acids and is produced by the parathyroid glands. Its best known role is to elevate blood calcium. The receptor for parathyroid hormone employs the G protein second messenger system.
Parathyroid hormone achieves its goal of raising the concentration of calcium in the blood by acting on three organs, the bones, the kidney and the small intestine.
Low to normal levels of PTH actually promote the activity of osteoblasts, which are the cells that form bone. This effect of PTH is necessary to maintain normal levels of bone turnover which allow remodelling in response to mechanical stresses but appears anomalous if parathyroid hormone is conceptualised only as existing to elevate blood calcium.
Higher levels of PTH initially cause the removal of calcium phosphate that is loosely attached to the surface of the bone. Later, osteoclasts are stimulated to resorb bone. In a rather circuitous arrangement, PTH cannot stimulate osteoclasts directly. Instead it induces osteoblasts to express RANKL (receptor activator for nuclear factor kappa B ligand) which in turn acts on RANK (the receptor for RANKL) which is present on osteoclast precursors. Binding of RANKL to RANK kicks the osteoclast precursors into gear as osteoclasts, which are programmed to resorb (destroy) bone.
Parathyroid hormone acts on the kidney to promote the reabsorption of calcium in the distal nephron. The reasborption of phosphate is reduced. The loss of phosphate decreases the quantity of calcium that is sequestered by binding to phosphate.
The combined action on the bone and kidney gives a co-ordinated response: more calcium is released from the bone and the kidney is instructed to make sure it hangs on to as much of it as possible.
The effect of PTH on the bowel is indirect. PTH stimulates the conversion of vitamin D to its active form in the kidney. The boost to activated vitamin D levels then promotes the absorption of calcium from the small intestine.
Vitamin D
Vitamin D is a fat soluble substance that is related to steroid hormones. Vitamin D can be acquired from the diet but can also be synthesised in the epidermis. The substrate for the cutaneous synthesis of vitamin D is 7-dehydrocholesterol. The conversion of 7-dehydrocholesterol into vitamin D requires ultraviolet B light. Wavelengths of ultraviolet light of 270-300nm can induce the conversion reaction, although the more effective range is narrower at 295-300nm and the ideal wavelength is 297nm. The reaction involves breaking one of the carbon-carbon bonds in the six carbon rings of the 7-dehydrocholesterol by the photons of the ultraviolet light, followed by a spontaneous isomerisation.
The form of vitamin D made in the skin or consumed in food is known as cholecalciferol and is inactive as a hormone. In order to become an effective hormone, it must undergo two hydroxylation reactions which transform it into 1,25-dihydroxycholecalciferol.
The addition of the hydroyxl group at position 25 is undertaken by the liver. The 25-hydroxycholecalciferol is stored in hepatocytes until it is needed, at which point it is released and sooner or later ends up in the renal proximal tubules. If the blood concentrations of calcium or phosphate are low, or the levels of parathyroid hormone are high, the tubular epithelial cells use a 1-alpha-hydroxylase to create 1,25-dihydroxycholecalciferol. If the 1-alpha-hydroxylase is not active a 24-hydroxylase enzyme can drag 25-hydroxycholecalciferol into its clutches to convert it into 24,25-dihydroxycholecalciferol, which is inert.
Dihydroxycholecalciferol is the active form of vitamin D. As befits a steroid-like hormone its receptor is located in the nucleus of cells. Dihydroxycholecalciferol increases the absorption of calcium and phosphate from the small intestine by promoting the synthesis of the protein calbindin. The renal tubules are induced to reabsorb more calcium and phosphate. Dihydroxycholecalciferol can potentiate the action of parathyroid hormone on bone and may directly stimulate osteoclasts.
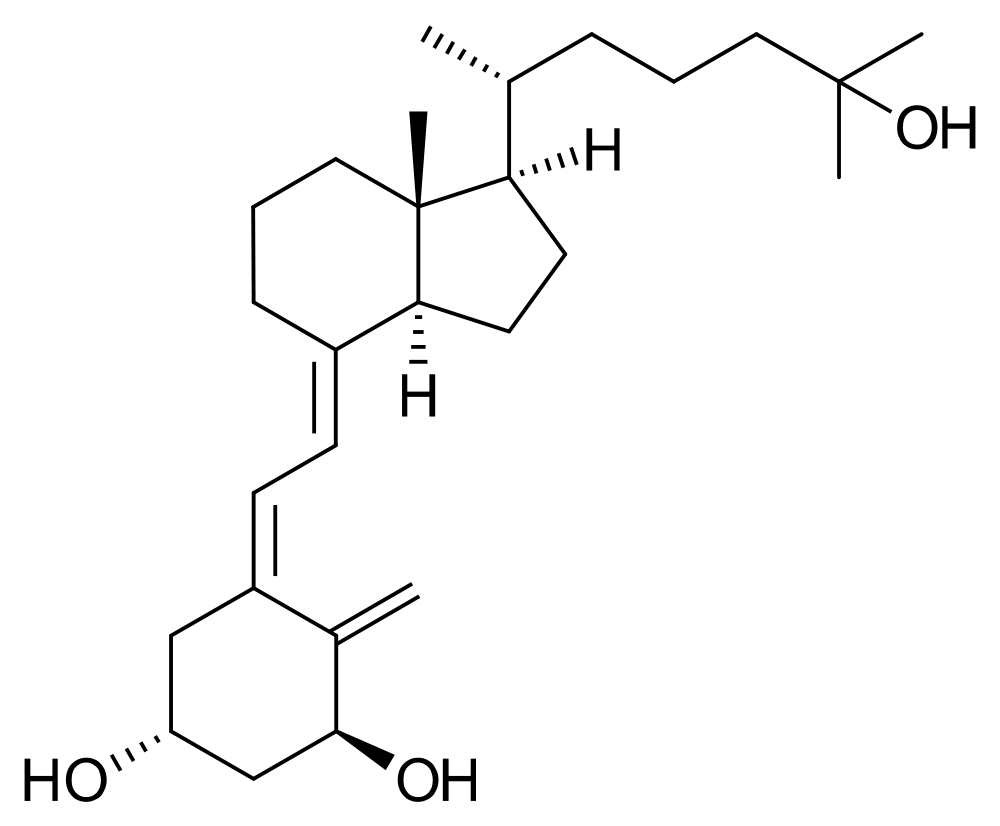
|
Calcitriol
Image courtesy of Wikipedia
|
Calcitonin
Whereas parathyroid hormone and vitamin D act to elevate blood calcium, calcitonin serves to reduce blood calcium and its release is therefore stimulated by an increase in blood calcium.
Calcitonin is synthesied by the C cells of the thyroid gland and is a peptide hormone which contains thirty-two amino acids. It inhibits bone resorption by osteoclasts and increases the activity of osteoblasts. The reabsorption of calcium by the kidney is inhibited.