Contents
Introduction
Much of the physiology of the female reproductive system relates to the menstrual cycle. The function of the menstrual cycle is to stimulate an oocyte to maturation and release from the ovary and to co-ordinate the maturation of the oocyte with changes in the endometrium which prepare the endometrium for implantation of a fertilized ovum. If implantation does not occur the endometrium breaks down and the cycle begins again.
Unlike sperm, which are generated throughout the reproductive life of a male, the supply of oocytes is limited in that they are all formed prior to birth (there is current debate as to whether this is absolute). This first form of the oocyte is called the
primordial follicle and after its creation further development is halted at an early stage of meiotic division.
Approximately 300,000 primordial follicles are present at birth.
The menstrual cycle is controlled by the hypothalamic-pituitary axis through the release of follicular stimulating hormone (FSH) and luteinising hormone (LH) by the pituitary gland. Various external factors have been suggested to be able to modulate the behaviour of the hypothalamic-pituitary axis and therefore alter the length of the menstrual cycle.
The age of onset of menstruation is known as menarche and occurs during puberty. The average age of menarche is around 12-13 years. The absence of menarche by 16 years is deemed to be abnormal and is referred to as primary amenorrhoea. Primary amenorrhoea may be the first symptom of an underlying disorder.
The lower end of the age range for menarche has been given as eight years; menarche that occurs before this age denotes precocious puberty and requires investigation for possible causes.
The standard length of the menstrual cycle is 28 days, although there can be variation between women and also between the cycles of an individual woman.
The menstrual cycle is divided into various sets of phases depending on whether the division is based upon changes in the endometrium, in the ovary or in hormonal levels. However, most schemes employ some variation on menstrual, proliferative, ovulatory and luteal.
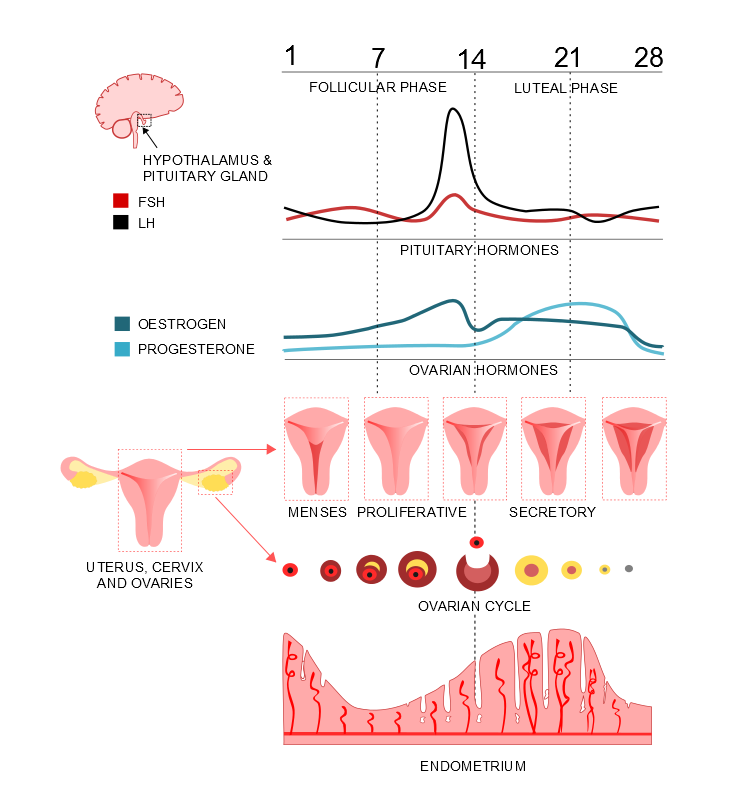
|
Diagram of some of the changes of the menstrual cycle
Diagram courtesy of Sarah Lee
|
Menstrual Phase
The menstrual phase is deemed to be the start of the menstrual cycle and therefore begins on day 1. The average duration is 3 to 5 days but the normal range is considered to be 2 to 7 days. The normal range for the volume of blood lost is 10-80ml
During the menstrual phase the endometrium is breaking down and being shed in the form of bleeding per vagina. This menstrual discharge occurs only in humans and some other primates; in most other placental mammals the breakdown of the endometrium is handled by local reabsorption rather than sloughing of the endometrium.
Proliferative Phase
The proliferative phase starts around day 5 and serves to allow a follicle to complete its maturation while the endometrium undergoes proliferation. It is driven mainly by FSH. There is also increased mucus production by the cervix.
Ovulatory Phase
The ovulatory phase of the menstrual cycle occupies days 13 to 16. The ovulatory phase is trigged by a surge in luteinising hormone at the end of the proliferative phase. This increase in LH stimulates the maturation of the oocyte into an ovum and also weakens the wall of the follicle in which the oocyte is contained.
The usual behaviour is for only one of the two ovaries to ovulate each month; bilateral ovulation is one of the mechanisms by which non-identical twins occur.
Luteal Phase
The luteal phase lasts from days 16 to 28. The endometrium switches to a secretory pattern that is receptive to the implantation of the blastocyst. Progesterone plays a key role in the luteal phase.
Towards the end of the luteal phase the levels of FSH, LH, oestrogen and progesterone drop and the removal of this support for the endometrium causes it to break down and slough, leading to menstruation and the start of another cycle.
Maturation of the Oocyte
At birth the ovaries are preloaded with all the primordial oocytes that they will ever possess. These primordial oocytes, located in primordial follicles, are around 30 to 50 micrometres in diameter and remain dormant until puberty and even then only a few are called into action during any one menstrual cycle.
Located within the centre of the follicle is the primordial oocyte itself, which is halted in meiosis at the first prophase. The lining of the primordial follicle is formed by granulosa cells. The granulosa cells can synthesise oestrogen and progesterone.
During the reproductive lifetime of the ovary, around two to three primordial follicles per day begin the process of maturation that may culminate in them forming an ovum. Exactly what triggers this process is unclear.
When a primordial oocyte starts to mature it becomes a
primary oocyte. The oocyte starts to express DNA that is related to further development of the oocyte. This results in the expression of receptors for FSH and the secretion of glycoproteins which form the zona pellucida around the primary oocyte. The zona pellucida is a clear layer that will allow sperm to bind to the ovum and fire their acrosomes to penetrate into the ovum itself. Development of a primary oocyte does not require FSH or LH. Primary oocytes have a diameter of 100 micrometers.
The next stage is the transformation to a
secondary oocyte / follicle. The primary oocyte acquires an external theca layer. This has two components, a theca externa and a theca interna, which are separated by a network of capillaries. The theca interna manufactures androstenedione, which can be used by the granulosa cells as a precursor for oestrogen. The role of the theca externa is one of structural support. The granulosa cell layer has increased to nine cells in thickness by this stage. Completion of the secondary follicle requires around 290 days after the primordial follicle starts of mature.
The
tertiary follicle follows the secondary follicle. The tertiary follicle is also known as the Graffian follicle. The follicle develops a fluid-filled cavity called the antrum and for this reason this stage of development is also referred to as the antral phase (the secondary follicle is sometimes called the preantral follicle). The granulosa and thecal cells increase in number in response to the increase in the size of the follicle that is due to the accumulation of the fluid. The granulosa cells differentiate into four subtypes which from the inside out are the corona radiata, cumulus oophorus, corona membrana and corona radiata.
The granulosa cells are responsive to and dependent on FSH by this juncture. The thecal cells are now responsive to LH.
At this stage the tertiary follicle is now finally ready to enter the menstrual cycle, 360 days after the maturation process first started. Most of the primordial follicles that began to mature at the same time have actually fallen by the wayside and undergone atresia, which is a form of apoptosis. Exactly what permits some follicles to stay the course and beat the others is unclear.
At entry to the menstrual cycle the tertiary follicle is around 2mm in diameter. Around six tertiary follicles enter the menstrual cycle together. FSH stimulates them to make oestradiol and inhibin. These feed back on the hypothalamic-pituitary axis to inhibit the release of FSH. As a consequence the follicles that have the lowest number of FSH receptors die. Ultimately, only one follicle has a sufficient density of FSH receptors to be able to survive on the prevailing concentration of FSH. This is the dominant follicle and has won the race to become an ovum.
The dominant follicle will progress to ovulation, by which stage it will be 20mm in size.
All is not over for the remains of the follicle once the ovum has been released in ovulation. Rather than immediately fading away it becomes a corpus luteum. The corpus luteum is a yellow structure that is derived from the thecal cells and granulosa cells. It synthesises progesterone which sustains the endometrium during the secretory phase of the endometrial cycle. The corpus luteum regresses towards the end of the secretory phase and the loss of the progesterone it secretes causes the endometrium to break down. The regressed corpus luteum may leave a structure known as a corpus albicans (dead language plural corpora albicantia).
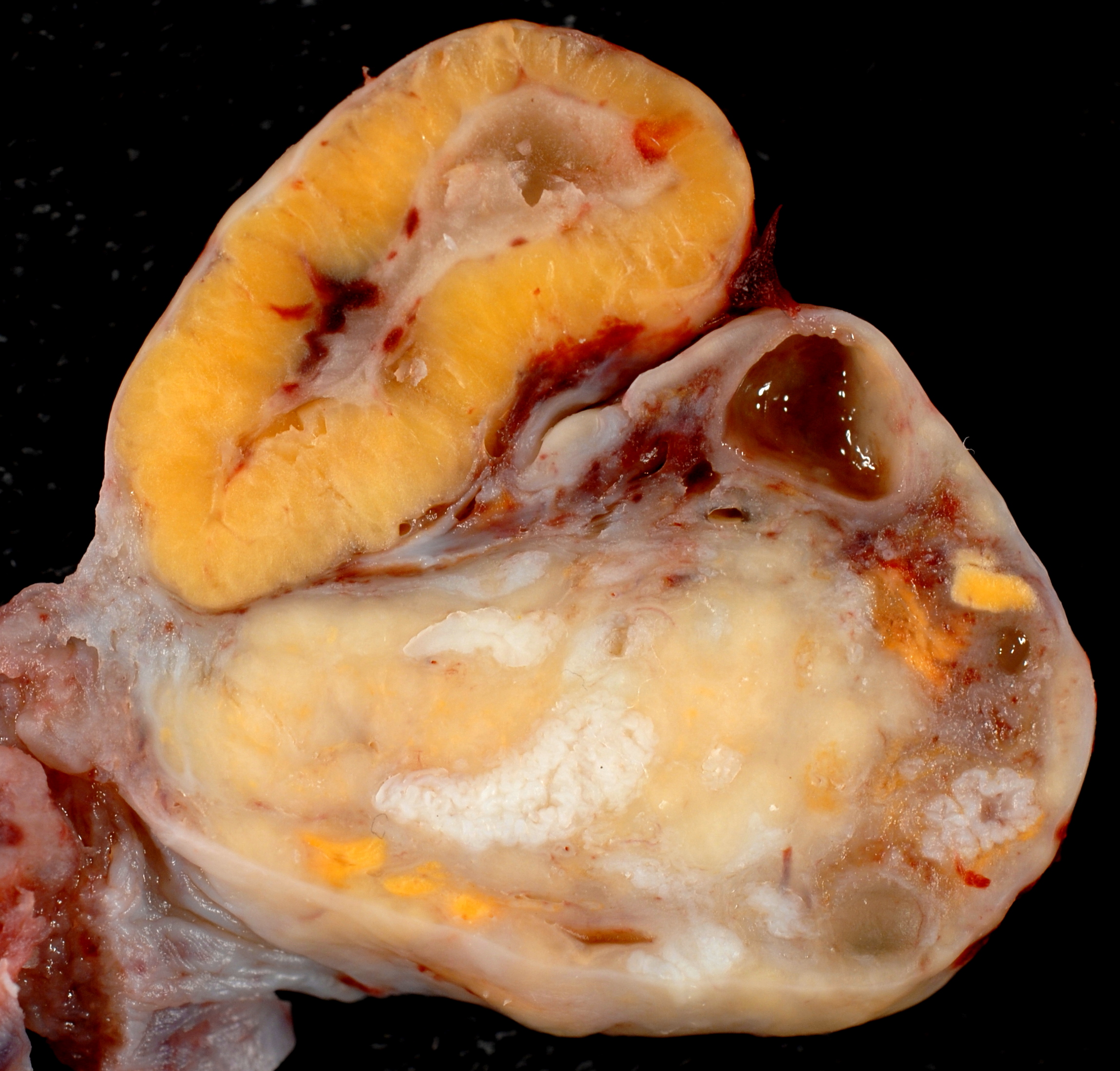
|
Photograph of a corpus luteum. A few corpora albicantia are present in the lower part of the ovary.
Image courtesy of Wikipedia
|
Hormonal Regulation of the Menstrual Cycle
The regulation of the menstrual cycle is accomplished primarily by follicular stimulating hormone, luteinising hormone, oestrogen and progesterone.
The levels of FSH rise slowly and modestly during the menstrual phase and this nudges along the competion between the six or so tertiary follicles that are competing to become the ovum. Once these teritary follicles start pumping out oestrogen and inhibin the release of FSH from the anterior pituitary gland falls a little while the follicles battle it out.
Once the dominant follicle is established it continues to secrete oestrogen. The hypothalamus has an alpha and beta oestrogen receptor. The alpha receptor mediates the negative feedback of oestrogen on the secretion of FSH. However, the beta receptor stimulates a positive feedback effect on the secretion of luteinising hormone. Thus, once the dominant follicle beats out the competition and celebrates by churning out high levels of oestrogen during the last part of the proliferative phase, the hypothalamic-pituitary axis is induced to release a surge of luteinising hormone and the luteinising hormone induces the tertiary follicle to mature to an ovum and permits ovulation to take place.
There is an FSH surge at the same time as the LH surge. This is not surprising as they are both under the control of gonadotropin releasing hormone (GnRH).
Once ovulation has occurred the corpus luteum secretes progesterone and this suppresses the release of both FSH and LH. This is bad news for the corpus luteum because it is dependent on LH for its continued existence. Towards the end of the secretory phase the concentration of LH is insufficient to sustain the corpus luteum which degenerates. This removes the progesterone block on FSH and LH secretion and the cycle can renew.
If the ovum becomes fertilised it needs to keep the corpus luteum alive in order to main the endometrium in its implantation-friendly secretory state. Therefore the blastocyst makes human chorionic gonadotropin which nourishes the corpus luteum until the placenta is sufficiently developed to take care of progesterone secretion itself.
Endometrial Changes
The endometrium undergoes cyclical changes during the menstrual cycle and it is the end point of these changes that are the actual event of menstruation.
The purpose of the changes in the endometrium is to convert the endometrium into a form that is receptive to the implantation of a fertilised ovum. Why the endometrium is not maintained in this state permanently is a matter for contemplation.
Once menstruation is completed all that remains of the endometrium is its basal layer. This undergoes proliferation, driven by oestrogen. Both the glands and the stroma proliferate. Mitotic activity is readily visible. Proliferative phase glands have smooth, ovoid contours, the cell nuclei are basally located and secretory activity is not present.
By the end of the proliferative phase the endometrium has increased in thickness from 1mm to 3mm. The spiral arteries have elongated, although do not really leave up to their name in terms of their shape at this juncture. The spiral arteries do not reach the upper third of the endometrium.
The endometrium now has two layers, the basal layer and the functional layer which was regenerated from the basal layer.
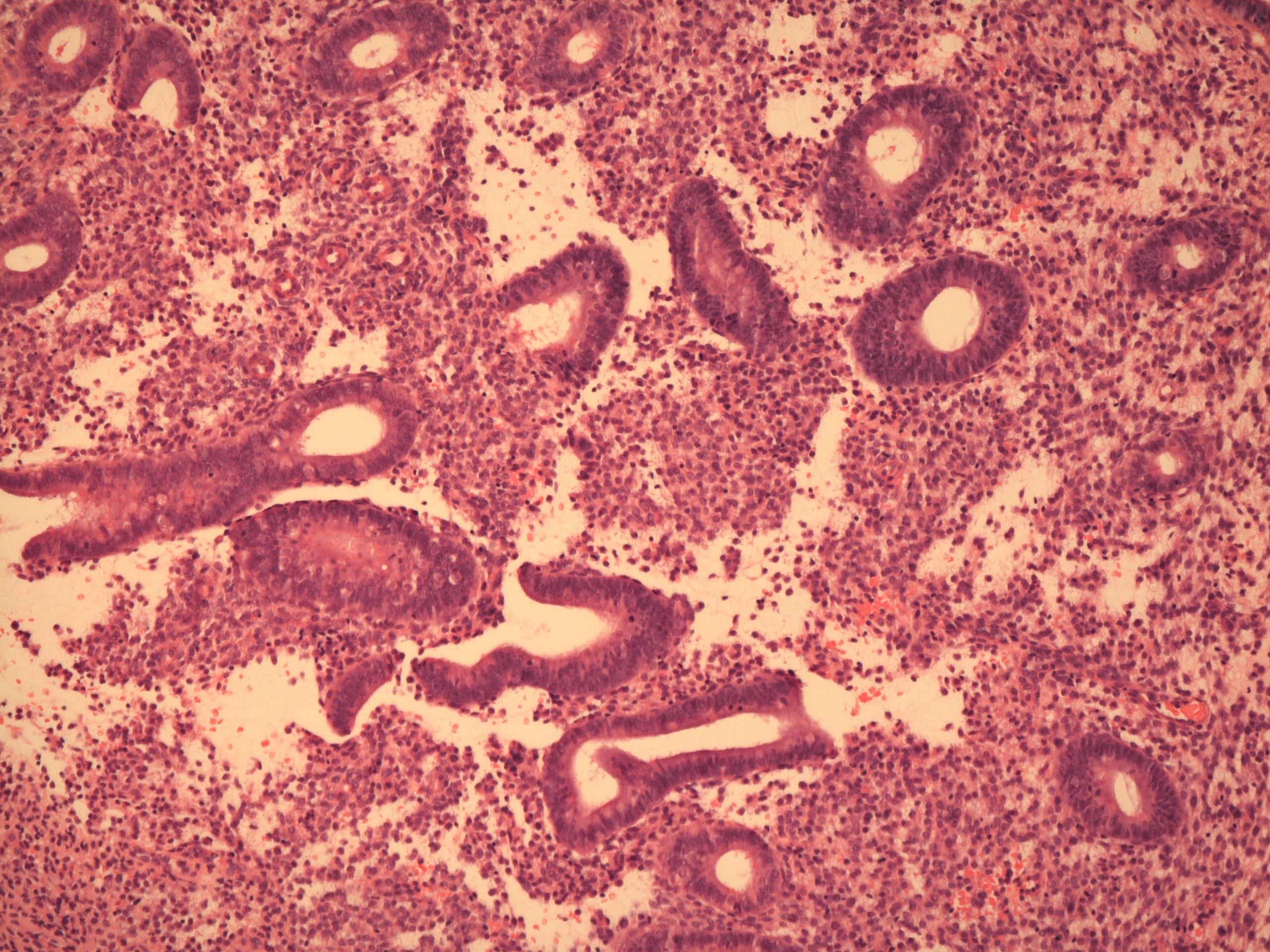
|
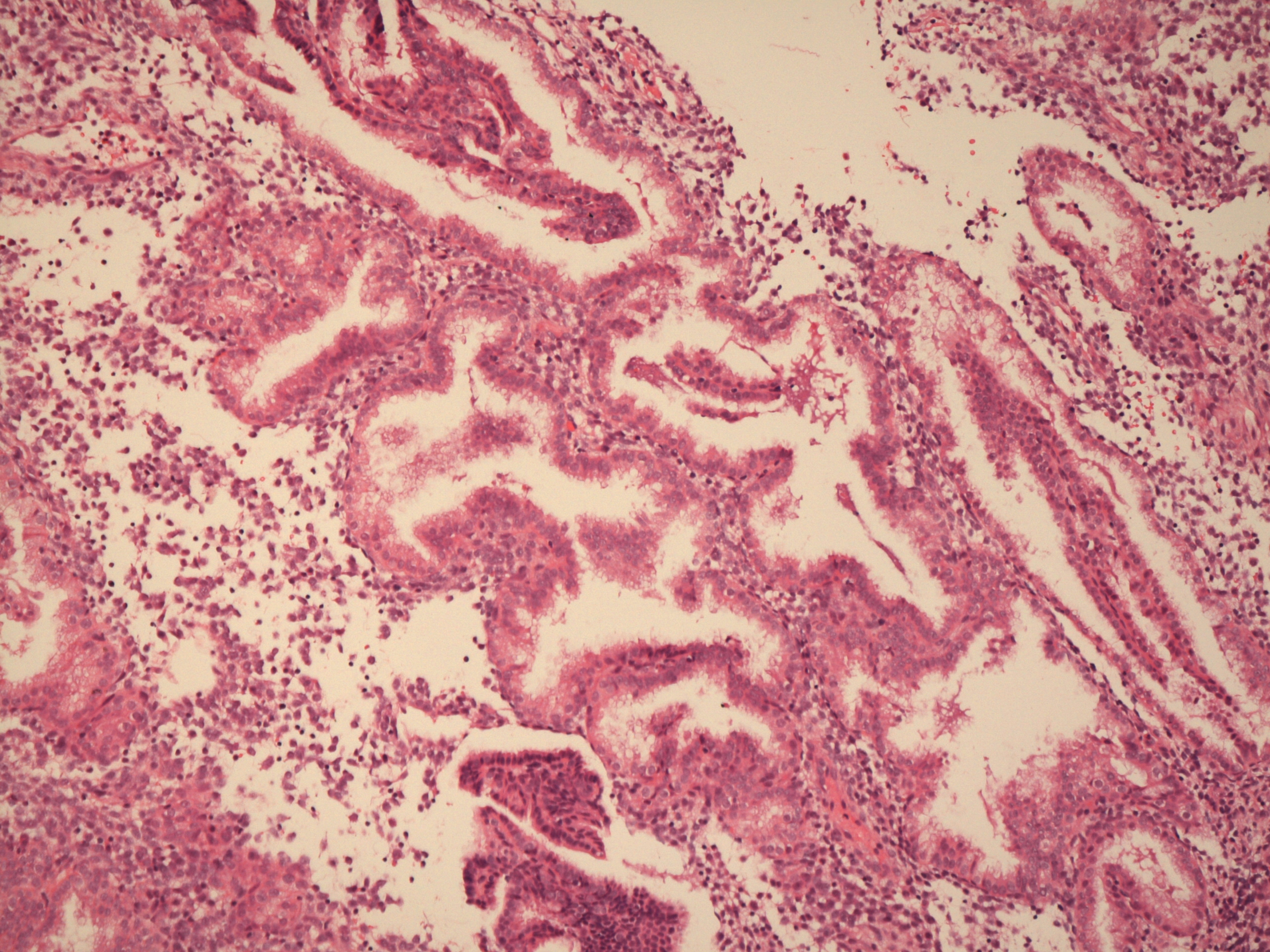
|
|
Proliferative endometrium
|
Secretory endometrium
|
The rise in progesterone levels that occurs after ovulation switches the endometrium into a secretory mode and the growth pattern from the hyperplasia of the proliferative phase to the hypertrophy of the secretory phase.
At the start of the secretory phase the cells of the glands have small vacuoles of glycogen situated just beneath their nucleus. These vacuoles are the first indication of the secretory phase.
The glands elongate and acquire convoluted luminal contours (the term corkscrew shaped may be used). The cytoplasm of the cells becomes clear as a consequence of their secretory activity. The lumens of the glands contains secretions. The stroma becomes oedematous. The spiral arteries lengthen and live up to their name; they extend almost all the way to the surface of the endometrium.
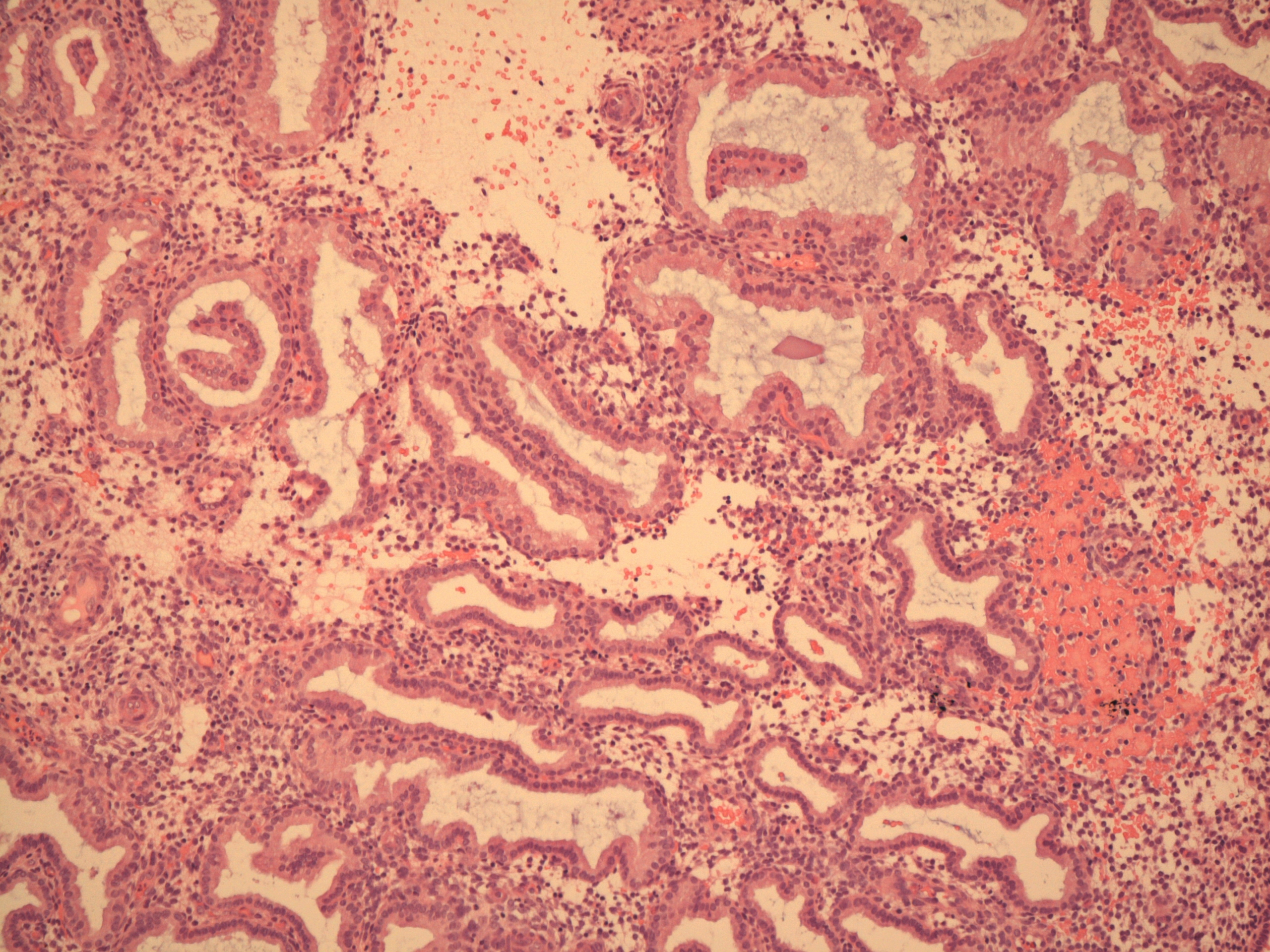
|

|
|
Early secretory phase endometrium
|
Late secretory phase endometrium
|
If implantation does not occur and progesterone levels fall the spiral arteries undergo episodes of contraction. This renders the functional layer of the endometrium ischaemic. The ischaemia causes the functional layer of the endometrium to become degenerate and eventually to slough off.
Decidual change is an alteration in the stromal cells that is dependent on progesterone and oestrogen and occurs if there is implantation of the blastocyst, but may also be encountered if there is sufficient increase in the concentration of progesterone or oestrogen for other reasons (for example drugs). Decidual stromal cells are large, pale, polyhedral cells that can look somewhat squamoid. Their main physiological role is during pregnancy when they form a layer which allows the placenta to separate from the uterus during the third stage of labour.
Cervical Changes
The cervix also undergoes changes during the menstrual cycle. For most of the menstrual cycle the cervix is firm and has a narrow canal. However, in the ovulatory period the canal widens, the cervix becomes softer and rises up, all of which facilitate the passage of sperm through the endocervical canal.
The mucus produced by the cervix also alters during the menstrual cycle. Immediately after menstruation the mucus is thick and acidic and is inhospitable to sperm and occludes the cervix. Around ovulation the mucus becomes thinner, more watery and less acidic and sperm-friendly.
Meiosis
Meiosis is a special form of cell division that is undertaken only by the germ cells of the gonads. It is the process by which gametes are formed and thus enables a diploid cell to give rise to haploid offspring.
Meiosis has two cell divisions. In the first meiotic division the cell reproduces each of its twenty-three pairs of chromosomes such that each chromosome now contains two identical copies of the strand of DNA; each copy is known as a chromatid. The two chromatids are linked at the centromere and it is this arrangement that give the classic 'X' shaped of a chromosome in diagrams of meiosis and mitosis.
The homologous chromosomes line up (the late Robin Williams in a performance at the 'Met' likened this to a square dance). Homologous chromosomes are the maternal and paternal copies of the same chromosome (for example, the maternal and paternal chromosome ones are homologous chromosomes but the maternal chromosome one and maternal chromosome two would not be homologous chromosomes, nor would be the maternal chromosome one and the paternal chromosome two). Each chromatid from the maternal homologous chromosome exchanges part of its sequence with one of the paternal homologous chromatids. This exchange of maternal and paternal material increases the genetic diversity that arises from sexual reproduction. The length of the chromatid that is swapped is relatively small and thus each chromatid remains predominantly maternal or paternal in composition.
The cell now has twenty three pairs of double-chromatid chromosomes and so needs to do something quite sharpish if it wishes to turn into haploid gametes. Having contributed to genetic diversity by the recombination of genes between chromatids, the first meiotic division now further mixes things up by dividing the cell into two daughter cells, each of which contains twenty-three recombined double-chromatid chromosomes. Any one of these double-chromatid chromosomes is now either primarily of maternal or paternal origin but not both. The allocation of maternal and paternal chromosomes to the daughter cells is random. These daughter cells are in effect haploid because they only contain twenty-three chromosomes (albeit that each chromsome is still in the double chromatid form).
The second meiotic division now just has to split the double-chromatid chromosomes into conventional single chain chromosomes. Each of the daughter cells arising from this second meiotic division will possess twenty-three unpaired chromosomes and is therefore haploid.
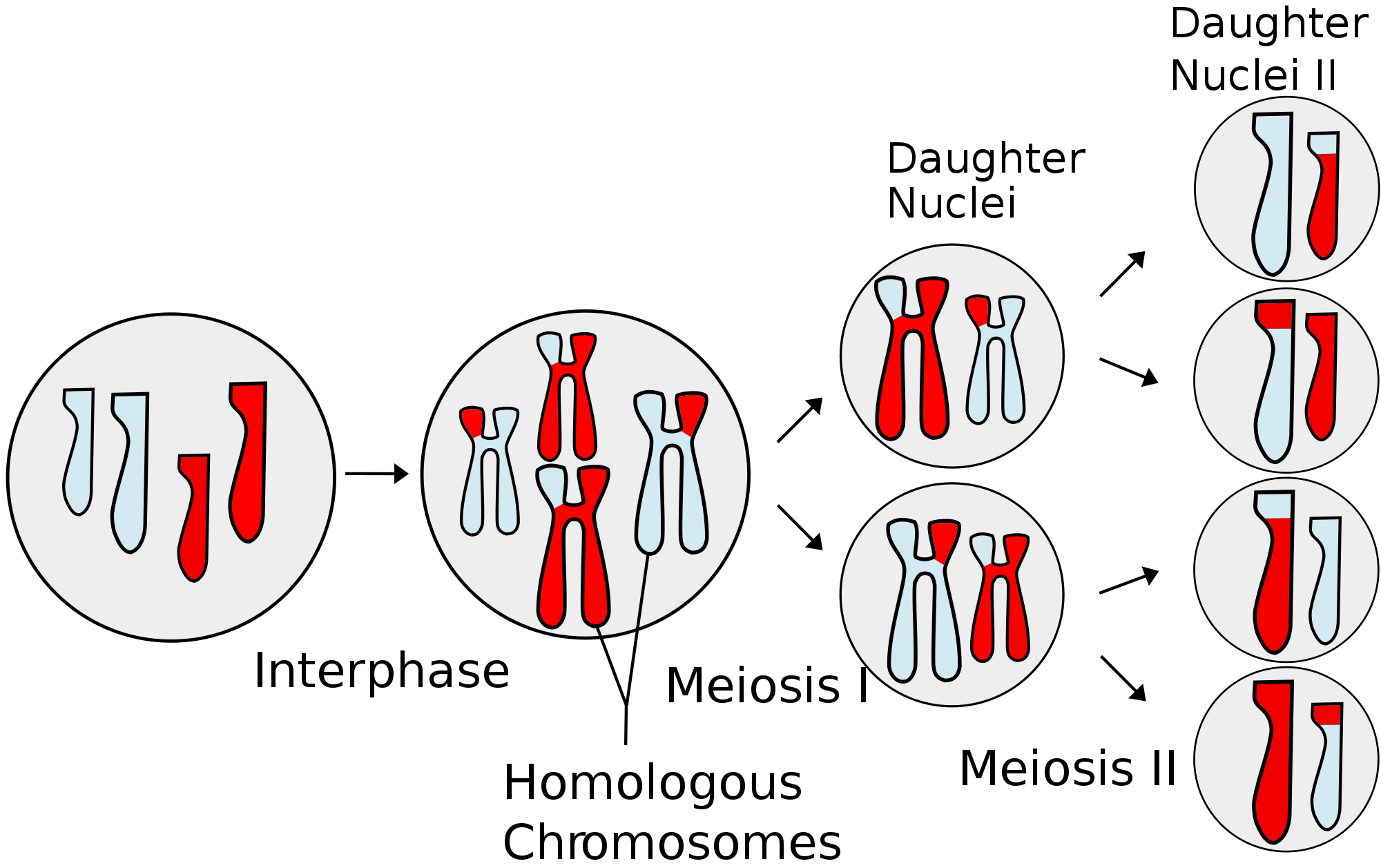
|
Diagram of meiosis
Diagram courtesy of Wikipedia
|
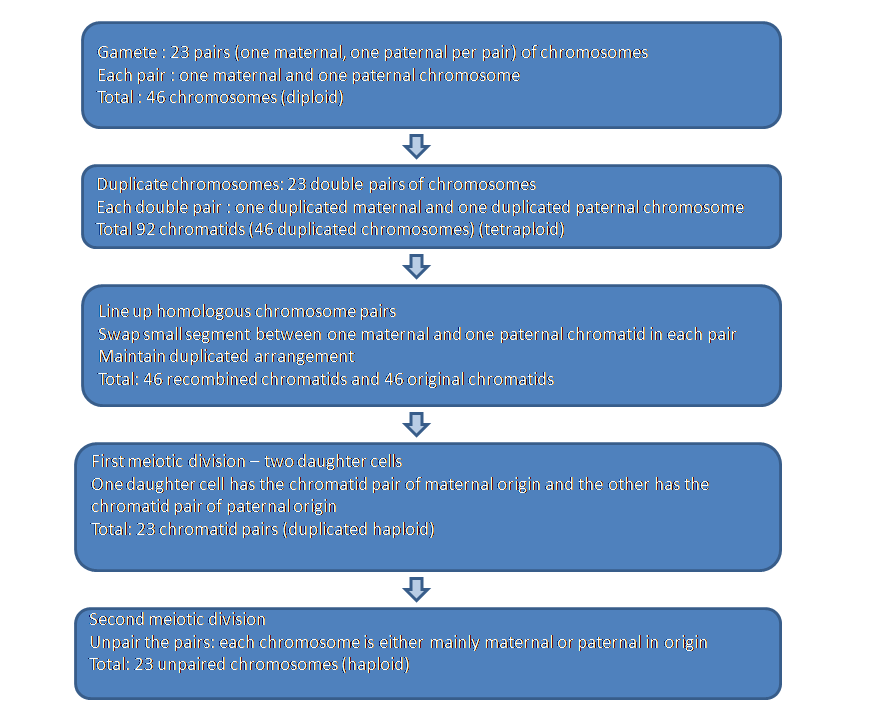
|
|
Flow chart of meiosis
|
Primordial oocytes have their meiotic division halted just after the recombination of material between homologous chromosomes. When the primordial oocyte matures to a secondary oocyte it completes the first meiotic division. However, one of the two daughter nuclei receives the vast majority of the cytoplasm. The other daughter cell becomes a small structure known as the first polar body, which then degenerates.
The second meiotic division is suspended in the secondary and tertiary oocyte and is actually only completed if fertilisation occurs. Once again, only one of the two daughter nuclei persists; the spare set of chromosomes is discarded as the second polar body. Thus, one primordial oocyte forms only one ovum. By contrast, the first stage cell of spermatogenesis, the spermatogonium, generates four spermatocytes.
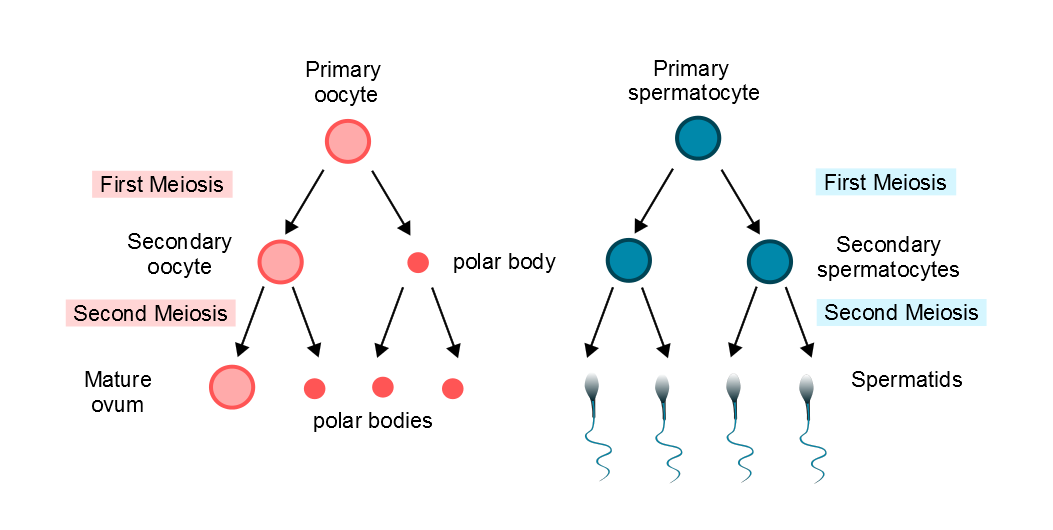
|
Diagram of oogenesis and spermatogenesis with regard to polar bodies
Diagram courtesy of Sarah Lee
|