Contents
Introduction
Acute inflammation is the body's rapid response system for infection and damage.
It forms part of the innate immune system and has cellular and humoral (chemical)
components.
Basic Features
The process of acute inflammation is characterised by four features that are traditionally
referred to as the cardinal signs of inflammation and were first described in the
First Century AD by Celsus.
- Redness
- Swelling
- Warmth
- Pain
Celsus was Roman and it is therefore understandable that he used Latin to describe
these features. The medical profession needs little encouragement to incorporate
Latin or Greek terms into its technical vocabulary, so for completeness the Latin
equivalents are rubor, tumor, calor and dolor respectively.
Around seventeen hundred years later, Virchow added a fifth feature, loss of function
(the Latin equivalent laesio functae, somewhat breaks up the rhythm of the first
four terms).
These four features are a direct consequence of the pathological events which occur
in acute inflammation and that are essential for the acute inflammatory response
to take place and function.
Acute inflammation causes increased blood flow to the affected region by dilatation
of the blood vessels (vasodilatation). This hyperaemia is responsible for the redness
and warmth and serves to increase the delivery of the various elements of the acute
inflammatory system to the site of the inflammation.
To facilitate the access of the agents of acute inflammation to the fray further,
capillary permeability increases, yielding
oedema.
Pain is the result of the release of various substances by the damaged tissue and
the inflammatory mediators. Pain encourages the organism to protect the inflamed
region and discourages movement, which may be important in some instances of healing.
In humans, pain may also prompt the individual to seek medical assistance to resolve
the problem.
Cellular Components

|
Neutrophils are the cells that are considered to be synonymous with acute
inflammation and the response to bacteria. They are phagocytic cells which possess
granules that contain various destructive substances such as lysozyme, myeloperoxidase
and enzymes that allow them to create hydrogen peroxide and oxygen based free radicals
which they use to kill the organisms they ingest.
The nucleus of a neutrophil has several lobes.
Neutrophils only have a lifespan of five days at the best of times. When called
into the chaos of acute inflammation they may surive exposure to the fray for only
a few minutes.
They constitute around 70% of white cells in the blood.
|

|
Like neutrophils, eosinophils are derived from myelopoietic cells in the
bone marrow. However, whereas neutrophils are infantry that engage in hand to hand
combat with infectious organisms, eosinophils are analagous to a form of artillery.
They contain numerous red granules that harbour assorted anti-microbial substances
which include histamine, eosinophil cationic protein and major basic protein. When
called into battle, eosinophils release these substances at their target and are
thus adapted to deal with large infectious organisms that are physically too big
to be phagocytosed.
Eosinophils are sometimes also thought of as part of the chronic inflammatory response.
Their numbers in the blood are much lower than those of neutrophils and they are
typically recruited to deal with specific problems that requires an eosinophilic
response, whereas neutrophil involvement is not usually selective.
|

|
Mast cells also execute their inflammatory function by discharging the contents
of their granules. These granules may not be appreciated with the H+E stain, but
can be demonstrated with the chloracetate esterase or toluidine blue stains. The
granules contain 5-hydroxytrypatmine and histamine. Degranulation is triggered when
multiple IgE molecules are bound to the surface of the mast cell, or if the mast
cell is damaged.
Unlike neutrophils, mast cells can live for several years.
In the peripheral blood, they are known as basophils. The basophil count in the blood is normally very low.
Basophils and mast cells are of myeloid origin.
|

|
Macrophages are versatile cells that are also involved in chronic inflammation.
In addition to killing organisms by phagocytosis and discharging the contents of
granules onto the phagocytosed organisms, they can engulf debris and small foreign
particles and present antigens to lymphocytes.
In the blood, macrophages are known as monocytes. They are derived from myeloid
cells in the bone marrow. Their nucleus is kidney bean shaped.
|
Chemical Components
Numerous chemicals are involved in acute inflammation and include multiple cytokines
such as assorted interleukins and tumour necrosis factors. However, there are two
particular chemical systems which require specific discussion.
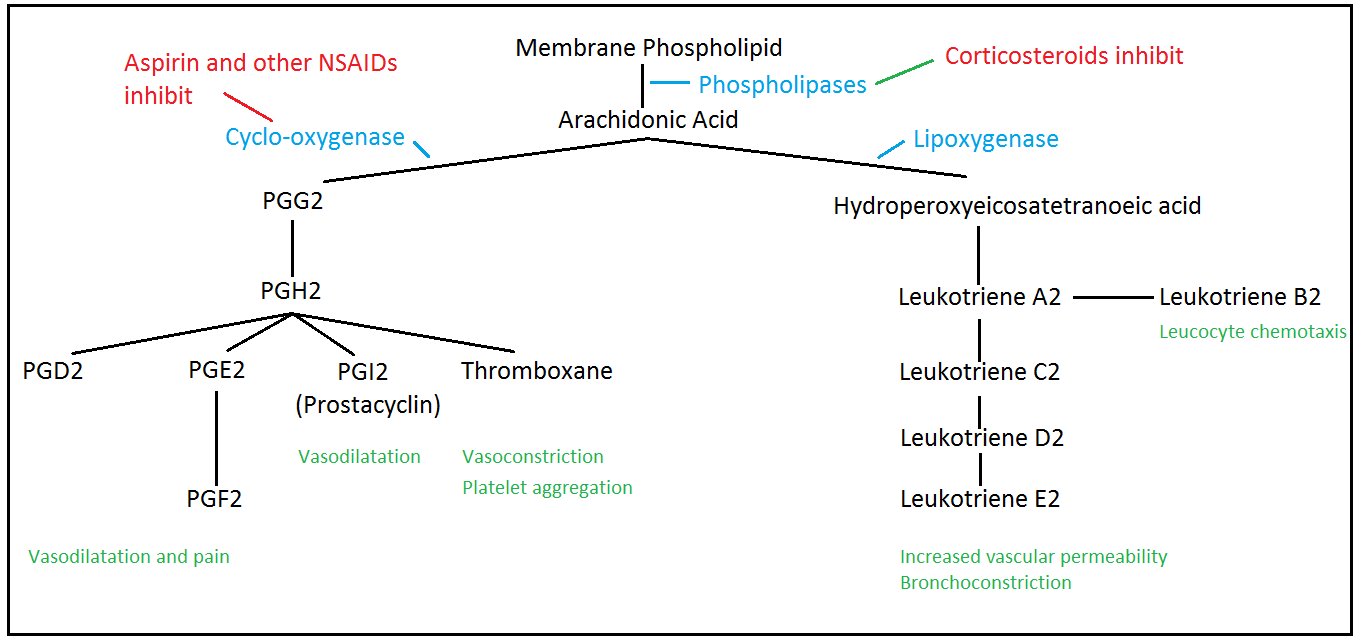
Arachidonic Acid
Arachidonic acid is a fatty acid that contains twenty carbon atoms. It is located
in cell membranes in combination with the phospholipids of the cell membrane. The
expression of the relevant phospholipases by a cell allows arachidonic acid to be
cleaved from its place of storage. Other enzymes then convert the arachidonic acid
into a variety of metabolites that have assorted effects on smooth muscle and leucocytes,
as well as stimulating pain receptors.
Cyclo-oxygenase is the enzyme that begins the conversion of arachidonic acid into
its assorted derivatives. It is inhibited by aspirin and other non-steroidal anti-inflammatory
drugs (for example, paracetamol) and this enables these drugs to exert their anti-inflammatory
and analgesic effects.
Complement
Complement is a very old element of the immune system in evolutionary biology
that is involved in the response to bacteria. It consists of a cascade of proteins,
each of which activates others until finally several components combine to form
the membrane attack complex. The membrane attack complex creates a hole in the cell
wall of a bacterium, thereby disrupting the osmotic integrity of the organism.
Some components of the complement system also act as signals to white cells and
help to co-ordinate the process of acute inflammation.
The presence of complement molecules on the surface of a bacterium makes it easier
for phagocytic leucocytes to engulf that organism (opsonisation).
The Cotrol of Acute Inflammation
Acute inflammation begins when either substances on the surface of bacteria
or intracellular contents released from damaged cells are detected.
Some bacterial surface components can activate elements of complement
and these activated components signal the start of the battle.
Intracellular substances
may either be inflammatory mediators in their own right (for example histamine in
mast cells) or be exclusively intracellular such that their presence outside a cell
can only be interpreted as denoting cell damage that requires an inflammatory response.
Endothelial cells have a vital role in enabling effective acute inflammation
to occur. In response to assorted chemicals (for example serotonin) they will contract
and thereby render the capillary more permeable. This allows circulating proteins
like complement and antibodies to enter the damaged tissue and also assists neutrophil
migration from the blood into the tissue. To facilitate neutrophil migration further,
the endothelial cells upregulate their synthesis of proteins on their luminal surface
that act as anchoring points to which neutrophils can bind. These cell adhesion
molecules allow neutrophils to attach to the wall of the capillary (margination),
then move into the tissue by extending pseudopodia. The multilobed nucleus of a
neutrophils makes it easier for the cell to squeeze through narrow gaps. Tumour
necrosis factor alpha is one substance that stimulates this upregulation by endothelial
cells.
Relaxation of vascular smooth muscle cells causes vasodilatation, which increases
blood flow to the affected tissue and delivers more leucocytes, complement and antibodies.
Prostaglandins and histamine are among the substances that promote vasodilatation.
Inflammatory mediators within the tissues provide a chemical signal and gradient
for neutrophils to follow. Thus, the process of chemotaxis allows neutrophils to
reach the place they are needed by homing in on these beacons (of which complement
components C3a and C5a are examples).
Once engaged in battle, neutrophils and macrophages will release other chemicals
that enhance the process of chemotaxis and reinforce the pro-inflammatory stance
that the endothelial cells have adopted.
Pus is a viscous liquid that is composed of numerous dead neutrophils and bacteria,
plus fibrin.
The acute inflammatory response is not only a localised phenomenon. Some interleukins
(IL-1 and IL-6) generated by the cells in the inflamed tissues stimulate increased
synthesis of neutrophils by the bone marrow. The inflammatory mediators also act
on the hypothalamus to resetthe core body temperate to a higher level. This fever is
believed to enhance the effectiveness of the immune system while impairing bacterial
enzymes. The synthesis of various proteins by the liver is also stimulated.
The acute inflammatory response is self-limiting. Once the infection has been eradicated
or the cell damage terminated, the stimuli that drive the inflammation will subside.
When this occurs, endothelial cell function returns to normal. Macrophages will
then clear up the mess left behind by the bacterial and cellular death and destruction.
Other cell types may also need to be called upon to help to restore the inflamed
tissue.